We are currently involved in five main topics:
1. Cooperative catalysis: We have recently demonstrated the design of several short peptoids incorporating both a catalytic metal-binding ligand and an organocatalyst, that act in a cooperative manner such that these peptoids could catalyze oxidation of primary alcohols almost 20 times faster than the segregated metal catalyst and organocatalyst. Our results represent the first example of such biomimetic activity by completely synthetic peptidomimetics. We are now developing chiral or helical peptoids in order to make this catalytic transformation asymmetric, aiming for the production of enantiopure alcohols and aldehydes.
2. Selective chelators for Cu(II), Zn(II) and Fe(III): Using helical peptoids and various synthetic techniques for the incorporation of metal-binding ligands with peptoids, we design
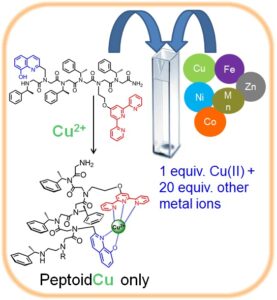
oligomeric chelators that should have high affinity and selectivity to one metal ion, namely Cu(II) or Zn(II), and two metal ions namely Cu(II) and Fe(III), Zn(II) and Fe(III) and Cu(II) and Zn(II). By incorporating both a N-, O-bidentate ligand and a N-tridentate ligand we have succeeded to generate a peptoid that is highly selective to Cu(II); this peptoid is able to coordinate only Cu(II), in an intramolecular fashion, from a solution mixture containing 20 equiv. of other metal ions e.g. Zn(II), Co(II), Fe(III) and Ni(II). To the best of our knowledge this is the first example of a metal chelator capable of such high selectivity. Moreover, this peptoid could bind two different metal ions, Cu(II) and Fe(III) in an intermolecular fashion, in specific sites along the peptoid by either kinetic or thermodynamic control.
3. Asymmetric catalysis: Peptoids have been designed as bio-mimetic materials including protein-protein interaction, metal binding and their utilization for medicinal applications. Despite their numerous functions, peptoid, even short oligomers are able to fold into well-defined secondary structures and enable incorporation of innumerable functional groups at specified N-positions along the peptoid backbone thus facilitating a wide range of chemical and structural diversity of the peptoid, which is limited in peptides and DNA. This feature highly eligible for effective stereo selective catalysts. Our group is focusing on helical peptoid catalysed asymmetric catalysis for C-C bond formation reaction (Aldol and Michael) and kinetic resolution of chiral amines. Along with probing the features of peptoid catalysts further and extending our studies with the relevant reactions.
4. New biomimetic catalysts for elctrochemical and photochemical water splitting: We are developing water-soluble inorganic clusters as well as peptoids bearing metal-binding ligands and other functional groups, to be used as electro- and photocatalysts for water splitting. We have succeeded to prepare a few complexes that show electrocatalytic activity regarding water splitting and we are now working towards their improvement.
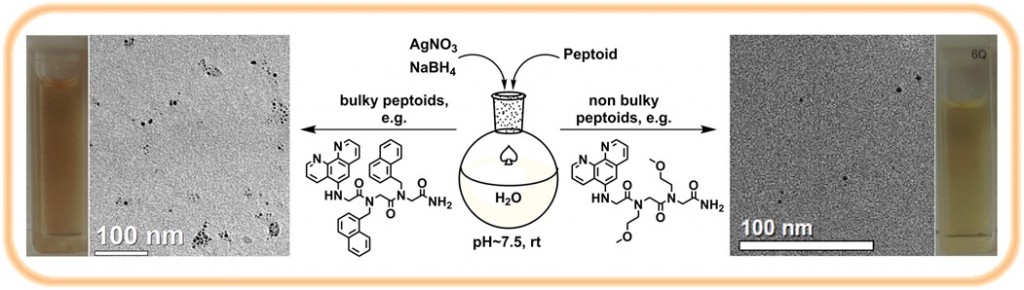
5. Metal nanoparticles assemblies mediated by peptoid oligomers: We are designing peptoids capable of binding and stabilizing metal nanoparticles (NPs), and of mediating their aggregation towards applications in chiral sensing and asymmetric heterogeneous catalysis. We have discovered that modifying the peptoid sequence, as well as the NPs binding ligand or the peptoid length has a great effect on the aggregation pattern of the NPs.
Why foldamers?
Folding of natural biopolymers is an important event, which is responsible for the creation of well-defined three-dimensional structures. In this process, specific arrangement of distinct functional groups enable them to operate collectively as one center, leading to their remarkable efficiency and selectivity in important functions such as catalysis, recognition and energy transfer. This relationship between structure and function has inspired the design of “foldamers” – oligomers that can fold upon non-covalent interactions to form well defined three-dimensional structures stable in solution. The class of foldamers we are currently interested in is peptidomimetic oligomers called peptoids.
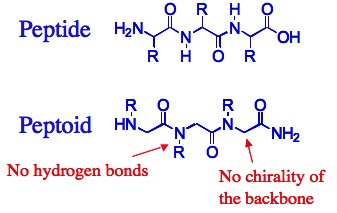
Why peptoids?
Compared with both biopolymers and synthetic polymers, peptoids have several advantages that make them better candidates for the design of functional materials, such as catalysts, sensors and drugs. First, ease of synthesis: peptoids are easily synthesized on solid support by the “submonomer” method – a repetitive two-step protocol in ambient conditions that typically requires short reaction times and no protection groups, involves a minimal number of purification steps and can be automated.
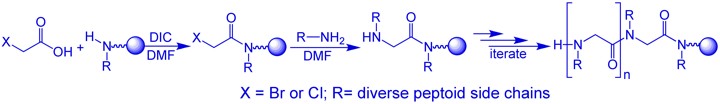
Second, versatility: the “sub-monomer” method employs primary amine synthons, therefore enabling the incorporation of innumerable functional groups (compared to only ~20 natural amino acids – the building blocks of peptides) at specified N-positions along the peptoid backbone thus facilitating the generation of peptoid oligomers possessing a wide range of chemical and structural diversity. Thus, properties such as chirality, solubility, hydrophilicity and hydrophobicity, can be tuned in a precise manner via introduction of different side chains at various positions. Third, structure stability: peptoids are robust materials, thermally stable and compatible with abiotic solvents, a wide range of pH conditions and various salt concentrations. In addition, peptoids are completely resistant to proteases, which rapidly cleave most peptide drugs in the blood and are not toxic.