Why foldamers?
Folding of natural biopolymers is an important event, which is responsible for the creation of well-defined three-dimensional structures. In this process, specific arrangement of distinct functional groups enable them to operate collectively as one center, leading to their remarkable efficiency and selectivity in important functions such as catalysis, recognition and energy transfer. This relationship between structure and function has inspired the design of “foldamers” – oligomers that can fold upon non-covalent interactions to form well defined three-dimensional structures stable in solution. The class of foldamers we are currently interested in is peptidomimetic oligomers called peptoids.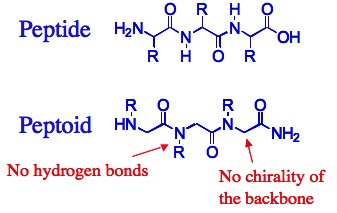
Why peptoids?
Compared with both biopolymers and synthetic polymers, peptoids have several advantages that make them better candidates for the design of functional materials, such as catalysts, sensors and drugs. First, ease of synthesis: peptoids are easily synthesized on solid support by the “submonomer” method – a repetitive two-step protocol in ambient conditions that typically requires short reaction times and no protection groups, involves a minimal number of purification steps and can be automated.
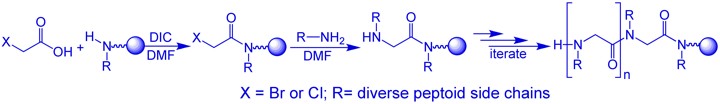
Second, versatility: the “sub-monomer” method employs primary amine synthons, therefore enabling the incorporation of innumerable functional groups (compared to only ~20 natural amino acids – the building blocks of peptides) at specified N-positions along the peptoid backbone thus facilitating the generation of peptoid oligomers possessing a wide range of chemical and structural diversity. Thus, properties such as chirality, solubility, hydrophilicity and hydrophobicity, can be tuned in a precise manner via introduction of different side chains at various positions. Third, structure stability: peptoids are robust materials, thermally stable and compatible with abiotic solvents, a wide range of pH conditions and various salt concentrations. In addition, peptoids are completely resistant to proteases, which rapidly cleave most peptide drugs in the blood and are not toxic.
We are currently interested in three main topics:
1. Interactions of Peptidomimetics with metal ions:
We synthesize peptidomimetics incorporating metal binding sites and investigate their interactions with metal ions by spectroscopic techniques such as UV and circular dichroism (CD). Our studies will be utilized to develop functional metallopeptoids with applications in asymmetric catalysis and drug design.
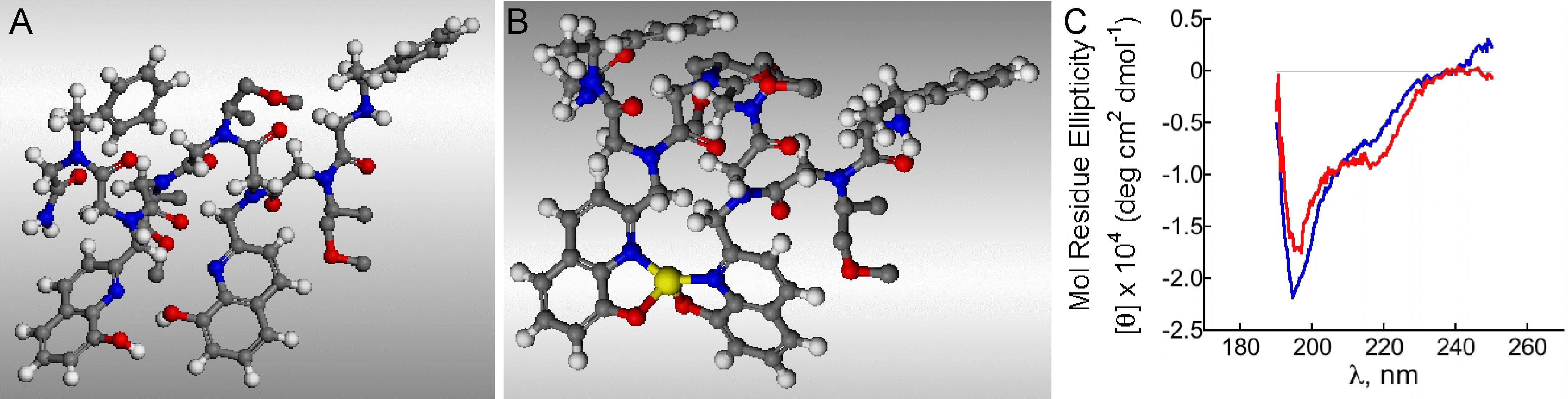
Energy-minimized structures of (A) metal-free non structured (non helical) peptoid and (B) The same peptoid becomes structured and adopts a helical structure after binding to a cobalt ion. Red: oxygen atoms, blue: nitrogen atoms, yellow: Co2+ ion. Geometry optimization was performed with the Universal Force Field in the Accelrys VS software modeling suite. (C) CD spectra of the non structured (non helical) peptoid before (blue) and after (red) binding to a cobalt ion. The helical structure is characterized by a double minima in the red spectrum, which is missing in the blue spectrum.
2. Aggregation of metal nanoparticles mediated by biomimetic oligomers:
We generate ensembles containing peptoids and metal nanoparticles by the synthesis of peptoids varied in chemical and structural properties and study them using spectroscopy and electron microscopy techniques. We also develop controlled aggregation approaches, for applications in chemical and/or biological sensing. The chiroptical properties of the new nanoparticle assemblies are evaluated towards applications in asymmetric catalysis.
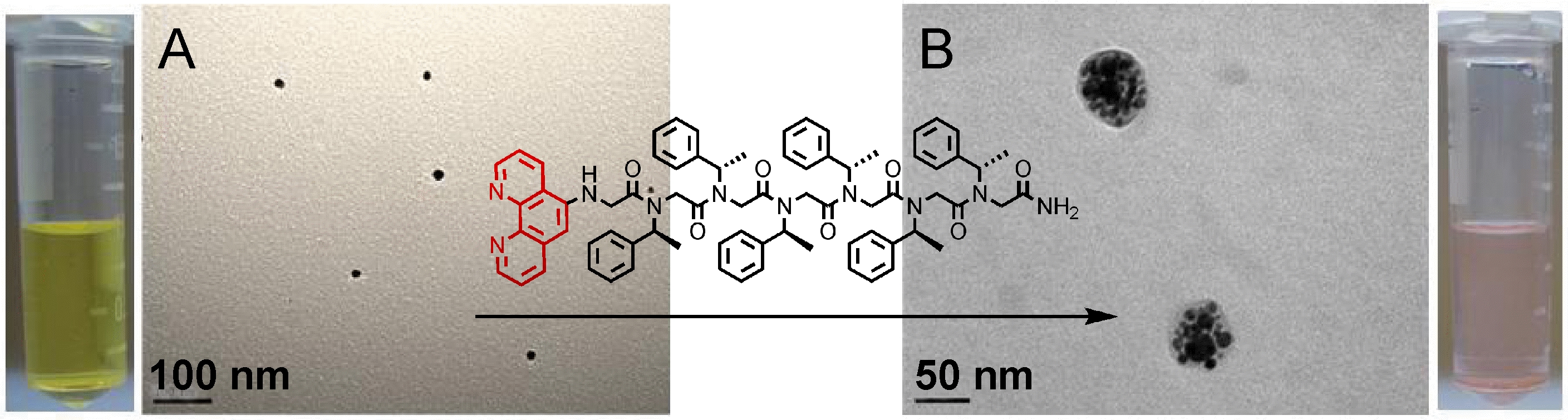 Ag(0) nanoparticles (A) before and (B) after peptoid functionalization.
Ag(0) nanoparticles (A) before and (B) after peptoid functionalization.
3. Microwave assisted chemical reactions on peptoids in the solid phase: We have recently described the incorporation of two bidentate metal-binding ligands via the [3+2] cycloaddition (“click”) reaction under MW irradiation on solid support. In addition, we have shown that we can incorporate an unprotected heterocyclic amine via a substitution reaction between the amine and a propyl chloride attached to the peptoid backbone, done under MW irradiation on solid support. We have also developed an efficient one step cyclization of peptoids under MW irradiation on solid support.
3. Biomimetic molecular catalysts for electrochemical and photochemical water splitting
One of the current worldwide efforts is the development of alternative energy technologies that generate clean sources of fuel. A specific challenge in this field is the economical production of hydrogen from water, the latter being a cheap, abundant and renewable resource. Our approach for the design of bio-inspired molecular catalysts for electrochemical and photochemical water splitting is based on the use of biomimetic oligomers in combination with unique metal complexes.

